Medidata Decentralized Clinical Trials Program
Medidata Solutions International Asia Pacific Pte. Ltd.
Medidata Decentralized Clinical Trials Program
Medidata Solutions International Asia Pacific Pte. Ltd.
 Back to Hall of Fame
Back to Hall of Fame
Medidata is the first and only company that unifies direct patient data
capture technology with study oversight and monitoring, redefining endto-
end decentralisation and transforming the site, sponsor, and patient
experience of decentralised clinical trials.
The Medidata Decentralized Clinical Trials (DCT) Program is the most
comprehensive set of unified, secure technologies that enable full
decentralisation across the clinical trial continuum. For the first time ever,
drug, vaccine, and medical device developers (sponsors) and contract
research organisations (CROs) can take advantage of the only platform
offering on the market which combines technology and workflows to
virtualise patient participation, tools that facilitate sponsor oversight
of patient safety and data quality, and direct-to-patient services that
enable at-home drug delivery.
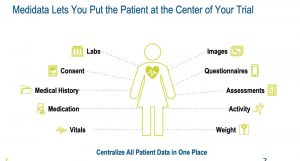
The Medidata DCT Program captures participant data remotely from
anywhere, at any time. It aggregates and transforms the data, monitors
the data to identify quality issues to mitigate risk and ensure patient
safety, and runs powerful analytics to draw new insights leading to
better outcomes for patients, researchers, sites, sponsors, and CROs.

Highlights
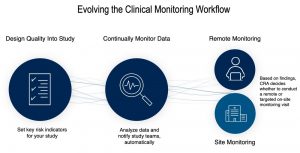
- Enables truly digital clinical oversight through embedded capabilities for risk identification, monitoring, and mitigation, optimising physical and virtual interaction with sites while maintaining patient safety and data quality.
- Allows for powerful workflows driven from patient-centric data, such as shipping investigational products directly to the patient and automated dosage adjustments.
- Provides the highest level of customisation of decentralising solutions based on study protocol design through the Trial Dial™ concept, and adjustments for everything from traditional onsite trials to fully decentralised models, and every hybrid trial design in between.
View Website
 Back to Hall of Fame
Back to Hall of Fame
Medidata is the first and only company that unifies direct patient data
capture technology with study oversight and monitoring, redefining endto-
end decentralisation and transforming the site, sponsor, and patient
experience of decentralised clinical trials.
The Medidata Decentralized Clinical Trials (DCT) Program is the most
comprehensive set of unified, secure technologies that enable full
decentralisation across the clinical trial continuum. For the first time ever,
drug, vaccine, and medical device developers (sponsors) and contract
research organisations (CROs) can take advantage of the only platform
offering on the market which combines technology and workflows to
virtualise patient participation, tools that facilitate sponsor oversight
of patient safety and data quality, and direct-to-patient services that
enable at-home drug delivery.
The Medidata DCT Program captures participant data remotely from
anywhere, at any time. It aggregates and transforms the data, monitors
the data to identify quality issues to mitigate risk and ensure patient
safety, and runs powerful analytics to draw new insights leading to
better outcomes for patients, researchers, sites, sponsors, and CROs.
Highlights
- Enables truly digital clinical oversight through embedded capabilities for risk identification, monitoring, and mitigation, optimising physical and virtual interaction with sites while maintaining patient safety and data quality.
- Allows for powerful workflows driven from patient-centric data, such as shipping investigational products directly to the patient and automated dosage adjustments.
- Provides the highest level of customisation of decentralising solutions based on study protocol design through the Trial Dial™ concept, and adjustments for everything from traditional onsite trials to fully decentralised models, and every hybrid trial design in between.