OBI-3424
OBI Pharma, Inc.
OBI-3424
OBI Pharma, Inc.
 Back to Hall of Fame
Back to Hall of Fame
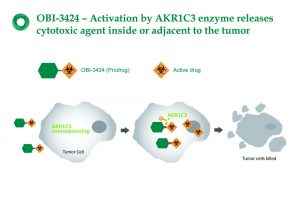
OBI-3424 is a first-in-class novel smallmolecule prodrug that selectively targets cancers overexpressing the enzyme aldo-keto reductase 1C3 (AKR1C3), and selectively releases a potent DNA alkylating agent in the presence of the AKR1C3 enzyme. This selective mode of activation distinguishes OBI-3424 from traditional alkylating agents, such as cyclophosphamide and ifosfamide, which are non-selective.
AKR1C3 overexpression has been documented in a number of treatmentresistant and difficult-to-treat cancers including hepatocellular carcinomas (HCC), castrate-resistant prostate cancer (CRPC), and acute lymphoblastic leukemia (ALL), including T-ALL. AKR1C3 is highly expressed in up to 15 solid and liquid tumors.
OBI PHARMA, INC. was granted the FDA Orphan Drug Designation for OBI-3424 for the Treatment of Hepatocellular Carcinoma (HCC) and Acute Lymphoblastic Leukemia (ALL). The Company conducted its first-inman clinical trial at the University of Texas M.D. Anderson Cancer Center ,The James Cancer Hospital and Solove Research Institute of Ohio State University.
OBI PHARMA, INC. holds worldwide rights for OBI-3424 with the exception of the following countries, whose rights are held by Ascentawits: China, Hong Kong, Macao, Taiwan, Japan, South Korea, Singapore, Malaysia, Thailand, Turkey, and India.

Highlights
OBI-3424 is a non-toxic prodrug which can be transformed into an effective chemotherapeutic drug via AKR1C3 in cancer cells. Consequently, the cancer cells can be destroyed directly without the normal cells being harmed.
The FDA has granted OBI-3424 Orphan Drug Designation for different treatments twice.
OBI is conducting its first-in-man clinical trial at the University of Texas M.D. Anderson Cancer Center, The James Cancer Hospital and Solove Research Institute of Ohio State University, two of America’s leading academic oncology research institutions.
Timing to market is late 2022, based on successful outcome results of clinical studies. Estimated peak territory revenue to be $1-2 Billion USD.
OBI-3424 can potentially enable patients to live a quality life during their cancer therapy in terms of its safety to protect the normal cells from being harmed.
View Website
 Back to Hall of Fame
Back to Hall of Fame
OBI-3424 is a first-in-class novel smallmolecule prodrug that selectively targets cancers overexpressing the enzyme aldo-keto reductase 1C3 (AKR1C3), and selectively releases a potent DNA alkylating agent in the presence of the AKR1C3 enzyme. This selective mode of activation distinguishes OBI-3424 from traditional alkylating agents, such as cyclophosphamide and ifosfamide, which are non-selective.
AKR1C3 overexpression has been documented in a number of treatmentresistant and difficult-to-treat cancers including hepatocellular carcinomas (HCC), castrate-resistant prostate cancer (CRPC), and acute lymphoblastic leukemia (ALL), including T-ALL. AKR1C3 is highly expressed in up to 15 solid and liquid tumors.
OBI PHARMA, INC. was granted the FDA Orphan Drug Designation for OBI-3424 for the Treatment of Hepatocellular Carcinoma (HCC) and Acute Lymphoblastic Leukemia (ALL). The Company conducted its first-inman clinical trial at the University of Texas M.D. Anderson Cancer Center ,The James Cancer Hospital and Solove Research Institute of Ohio State University.
OBI PHARMA, INC. holds worldwide rights for OBI-3424 with the exception of the following countries, whose rights are held by Ascentawits: China, Hong Kong, Macao, Taiwan, Japan, South Korea, Singapore, Malaysia, Thailand, Turkey, and India.
Highlights
OBI-3424 is a non-toxic prodrug which can be transformed into an effective chemotherapeutic drug via AKR1C3 in cancer cells. Consequently, the cancer cells can be destroyed directly without the normal cells being harmed.
The FDA has granted OBI-3424 Orphan Drug Designation for different treatments twice.
OBI is conducting its first-in-man clinical trial at the University of Texas M.D. Anderson Cancer Center, The James Cancer Hospital and Solove Research Institute of Ohio State University, two of America’s leading academic oncology research institutions.
Timing to market is late 2022, based on successful outcome results of clinical studies. Estimated peak territory revenue to be $1-2 Billion USD.
OBI-3424 can potentially enable patients to live a quality life during their cancer therapy in terms of its safety to protect the normal cells from being harmed.