BRM421, AN INNOVATIVE REGENERATIVE PEPTIDE FOR DRY EYE DISEASE
BRIM BIOTECHNOLOGY, INC.
BRM421, AN INNOVATIVE REGENERATIVE PEPTIDE FOR DRY EYE DISEASE
BRIM BIOTECHNOLOGY, INC.
 Back to Hall of Fame
Back to Hall of Fame
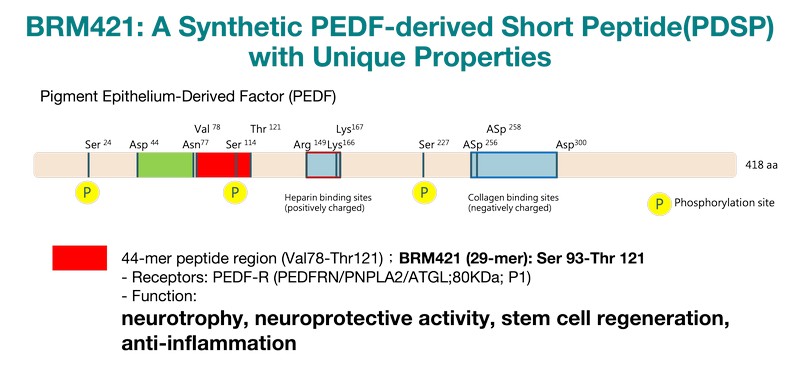
BRM421 is a first-in-class ophthalmic solution specifically developed for the treatment of Dry Eye Disease (DED). It comprises a synthetic peptide made of 29 amino acids, derived from Pigment Epithelium-Derived Factor (PEDF), which has neurotrophic and anti-inflammatory effects that support tissue regeneration on the eye’s surface.
BRM421 stands out by uniquely activating corneal limbal stem cells, boosting their growth and differentiation to accelerate corneal repair. It also promotes goblet cell growth, improves tear quality, and directly benefits DED patients.
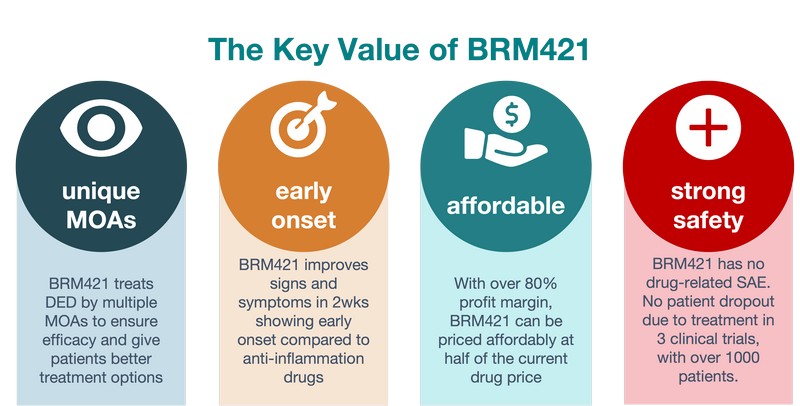
The preliminary clinical trial has shown that BRM421 delivers effective results within just two weeks, which is faster than most current therapies. Additionally, BRM421 has an excellent safety record, with no patient withdrawals due to BRM421-related adverse effects across all three clinical trials. Its affordable pricing makes BRM421 an accessible and promising choice for patients seeking fast, effective, and affordable relief from DED.

Highlights
- BRM421 stimulates limbal stem cells for corneal repair and facilitates goblet and meibomian gland restoration.
- Demonstrates effectiveness within two weeks, significantly faster than most of the approved therapies.
- Offers cost-effective treatment with a strong profit margin, providing significant savings for patients.
- No patient withdrawals due to BRM421-related adverse effects in clinical trials, with side effects significantly lower than most approved treatments.
View Website
 Back to Hall of Fame
Back to Hall of Fame
BRM421 is a first-in-class ophthalmic solution specifically developed for the treatment of Dry Eye Disease (DED). It comprises a synthetic peptide made of 29 amino acids, derived from Pigment Epithelium-Derived Factor (PEDF), which has neurotrophic and anti-inflammatory effects that support tissue regeneration on the eye’s surface.
BRM421 stands out by uniquely activating corneal limbal stem cells, boosting their growth and differentiation to accelerate corneal repair. It also promotes goblet cell growth, improves tear quality, and directly benefits DED patients.
The preliminary clinical trial has shown that BRM421 delivers effective results within just two weeks, which is faster than most current therapies. Additionally, BRM421 has an excellent safety record, with no patient withdrawals due to BRM421-related adverse effects across all three clinical trials. Its affordable pricing makes BRM421 an accessible and promising choice for patients seeking fast, effective, and affordable relief from DED.


Highlights
- BRM421 stimulates limbal stem cells for corneal repair and facilitates goblet and meibomian gland restoration.
- Demonstrates effectiveness within two weeks, significantly faster than most of the approved therapies.
- Offers cost-effective treatment with a strong profit margin, providing significant savings for patients.
- No patient withdrawals due to BRM421-related adverse effects in clinical trials, with side effects significantly lower than most approved treatments.


