CLINICAL DATA STUDIO
MEDIDATA SOLUTIONS INTERNATIONAL ASIA PACIFIC PTE. LTD.
CLINICAL DATA STUDIO
MEDIDATA SOLUTIONS INTERNATIONAL ASIA PACIFIC PTE. LTD.
 Back to Hall of Fame
Back to Hall of Fame
Clinical trials are becoming increasingly complex, which could lead to lower performance, higher failure rates, longer cycle times, and poorer data quality. On one hand, there is a focus on designing and executing innovative, patient-friendly trials, including new ways to collect richer patient data sets. On the other hand, managing the growing volume of data, data sources, and complexity has introduced new challenges, such as a 27% increase in endpoints and a 10% increase in trial complexity. However, despite these growing risks, only 57% of sponsors or CROs have adopted Risk-Based Quality Management (RBQM).
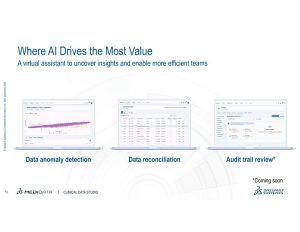
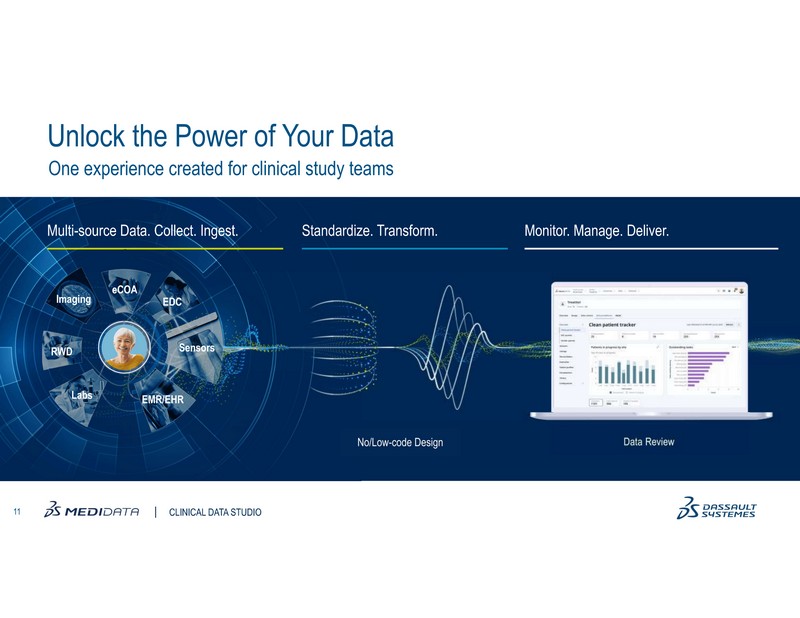
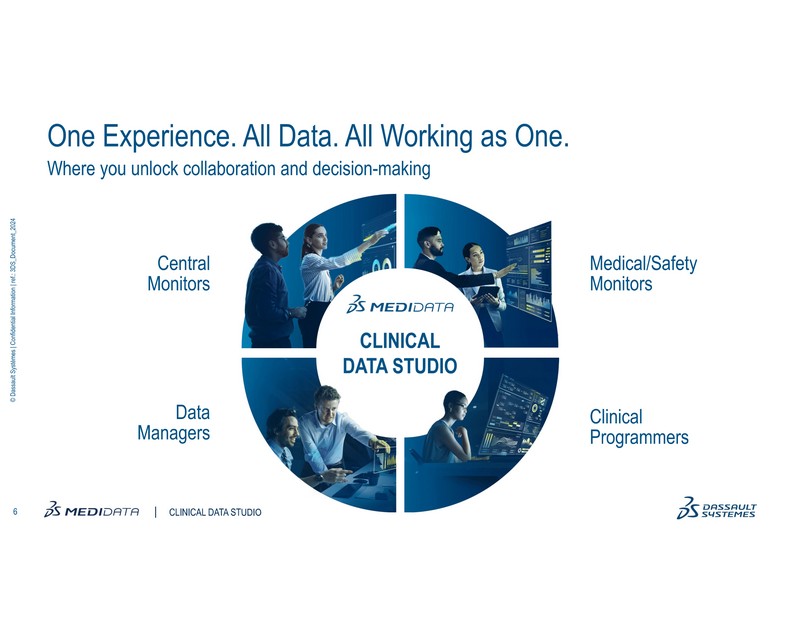
To help overcome these challenges, Medidata Clinical Data Studio, a transformative AI-powered data management and quality experience was launched in June. This technology offers a unified and seamless experience by enabling multi-source data integration, transformation, and analysis for diverse stakeholders.
Built with a user-friendly, low-code environment, it leverages AI to streamline data aggregation, standardization, and management workflows, allowing multiple users to act on real-time data to reduce burden, shorten review timelines, increase quality, reduce risk, and improve patient safety.

Highlights
- Assisted by AI and automation, Clinical Data Studio enables data managers to shorten review cycle times by up to 80% per review cycle and grants clinical data management access to review all clinical trial data in one place.
- Clinical Data Studio offers a low-code environment that makes data ingestion, standardization, and transformation accessible to clinical data managers, central monitors, and medical monitors for review and analytics.
- Clinical Data Studio delivers remarkable results: 90% reduction in listing generation, 80% reduction in data review cycle time, 85% reduction in KRI configuration time and as little as 3 days to get it up and running.
View Website
 Back to Hall of Fame
Back to Hall of Fame
Clinical trials are becoming increasingly complex, which could lead to lower performance, higher failure rates, longer cycle times, and poorer data quality. On one hand, there is a focus on designing and executing innovative, patient-friendly trials, including new ways to collect richer patient data sets. On the other hand, managing the growing volume of data, data sources, and complexity has introduced new challenges, such as a 27% increase in endpoints and a 10% increase in trial complexity. However, despite these growing risks, only 57% of sponsors or CROs have adopted Risk-Based Quality Management (RBQM).
To help overcome these challenges, Medidata Clinical Data Studio, a transformative AI-powered data management and quality experience was launched in June. This technology offers a unified and seamless experience by enabling multi-source data integration, transformation, and analysis for diverse stakeholders.
Built with a user-friendly, low-code environment, it leverages AI to streamline data aggregation, standardization, and management workflows, allowing multiple users to act on real-time data to reduce burden, shorten review timelines, increase quality, reduce risk, and improve patient safety.


Highlights
- Assisted by AI and automation, Clinical Data Studio enables data managers to shorten review cycle times by up to 80% per review cycle and grants clinical data management access to review all clinical trial data in one place.
- Clinical Data Studio offers a low-code environment that makes data ingestion, standardization, and transformation accessible to clinical data managers, central monitors, and medical monitors for review and analytics.
- Clinical Data Studio delivers remarkable results: 90% reduction in listing generation, 80% reduction in data review cycle time, 85% reduction in KRI configuration time and as little as 3 days to get it up and running.