MCIRBP™-19
GREENYN BIOTECHNOLOGY CO., LTD.
MCIRBP™-19
GREENYN BIOTECHNOLOGY CO., LTD.
 Back to Hall of Fame
Back to Hall of Fame
The demand for blood sugar health supplements has long been an untapped market. In recent years, mcIRBP-19™ has emerged as a rising star across Asia, driving significant business opportunities in the sub-health market.
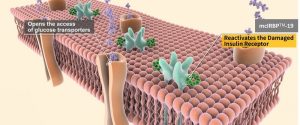
Developed over a decade, mcIRBP-19™ is a small molecular sequenced peptide with a molecular weight of just 2,162 Da. With its unique mechanism of action, it is often referred to as "plant insulin." Backed by seven scientific publications validating its efficacy and mechanisms, mcIRBP-19™ has garnered recognition from major global invention awards as a health supplement that supports blood sugar management.
In production, the adoption of supercritical extraction technology and the implementation of a sustainable carbon cycle process not only enhance extraction efficiency but also ensure solvent-free manufacturing for safe and effective results. To cater to the unique needs of different markets and support localized promotion efforts, a dual-brand marketing strategy has been implemented, leading to the development of Insumate® and IRK-19®.
Insumate, a patented sequenced 19-peptide derived from bitter melon extract, is currently the only bitter melon peptide to have received a No Objection Letter (NDIN No. 1256) from the U.S. FDA under the New Dietary Ingredient (NDI) safety review. Its exceptional scientific foundation and safety profile have garnered widespread attention from leading global brands.



Highlights
- The patented sequenced 19-peptide mcIRBP™-19 fills the gap in blood sugar regulation health supplements, holding significant market potential and future implications.
- mcIRBP™-19, often referred to as "plant insulin," features a distinct mechanism of action supported by research and peer-reviewed publications.
- This innovative process replaces conventional low-yield, high-carbon-emission extraction methods with optimized conditions for supercritical fluid extraction, enabling carbon recovery and sustainable recycling.
- mcIRBP™-19 has been developed into two distinct brands, Insumate® and IRK-19®, to enhance customer engagement and broaden marketing coverage.
- mcIRBP™-19 is the world’s first bitter melon extract to receive a No Objection Letter (NDIN #1256) from the U.S. FDA under the New Dietary Ingredient (NDI) framework, with clinically verified efficacy and safety.
View Website
 Back to Hall of Fame
Back to Hall of Fame
The demand for blood sugar health supplements has long been an untapped market. In recent years, mcIRBP-19™ has emerged as a rising star across Asia, driving significant business opportunities in the sub-health market.
Developed over a decade, mcIRBP-19™ is a small molecular sequenced peptide with a molecular weight of just 2,162 Da. With its unique mechanism of action, it is often referred to as "plant insulin." Backed by seven scientific publications validating its efficacy and mechanisms, mcIRBP-19™ has garnered recognition from major global invention awards as a health supplement that supports blood sugar management.
In production, the adoption of supercritical extraction technology and the implementation of a sustainable carbon cycle process not only enhance extraction efficiency but also ensure solvent-free manufacturing for safe and effective results. To cater to the unique needs of different markets and support localized promotion efforts, a dual-brand marketing strategy has been implemented, leading to the development of Insumate® and IRK-19®.
Insumate, a patented sequenced 19-peptide derived from bitter melon extract, is currently the only bitter melon peptide to have received a No Objection Letter (NDIN No. 1256) from the U.S. FDA under the New Dietary Ingredient (NDI) safety review. Its exceptional scientific foundation and safety profile have garnered widespread attention from leading global brands.


Highlights
- The patented sequenced 19-peptide mcIRBP™-19 fills the gap in blood sugar regulation health supplements, holding significant market potential and future implications.
- mcIRBP™-19, often referred to as "plant insulin," features a distinct mechanism of action supported by research and peer-reviewed publications.
- This innovative process replaces conventional low-yield, high-carbon-emission extraction methods with optimized conditions for supercritical fluid extraction, enabling carbon recovery and sustainable recycling.
- mcIRBP™-19 has been developed into two distinct brands, Insumate® and IRK-19®, to enhance customer engagement and broaden marketing coverage.
- mcIRBP™-19 is the world’s first bitter melon extract to receive a No Objection Letter (NDIN #1256) from the U.S. FDA under the New Dietary Ingredient (NDI) framework, with clinically verified efficacy and safety.