myMedidata - The Industry’s First Patient Portal Platform
Medidata Solutions International Asia Pacific Pte Ltd
myMedidata - The Industry’s First Patient Portal Platform
Medidata Solutions International Asia Pacific Pte Ltd
 Back to Hall of Fame
Back to Hall of Fame
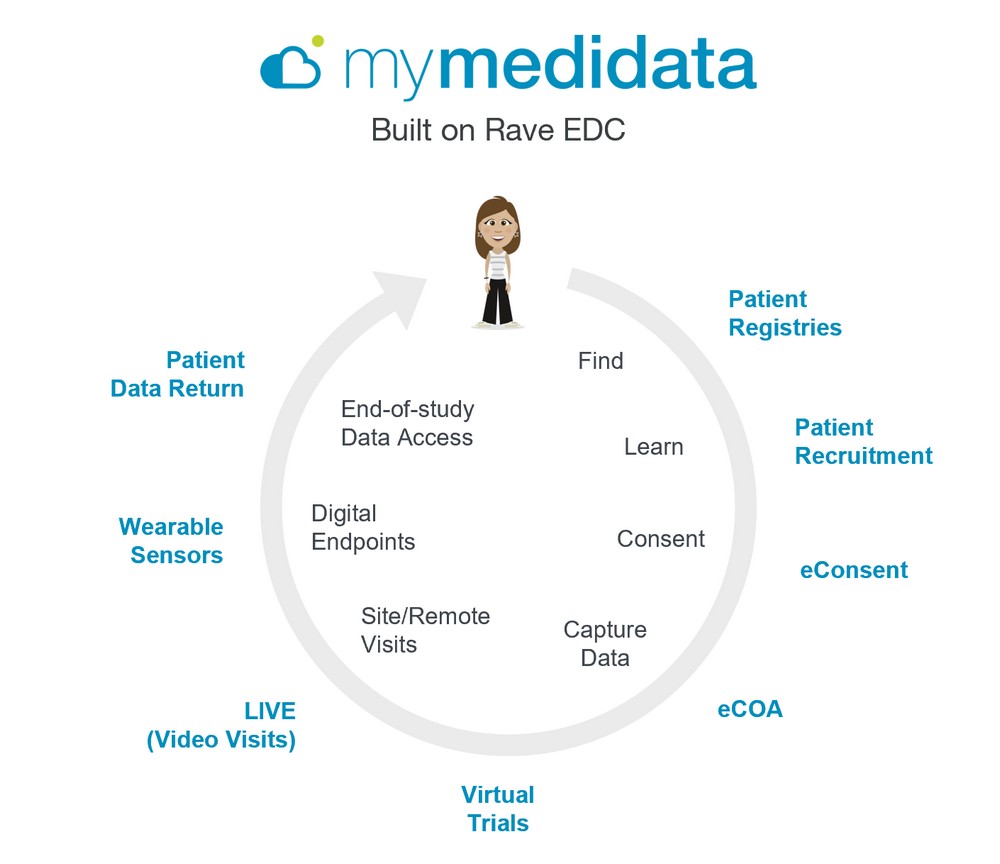
Medidata’s patient portal — myMedidata — is a
single-destination platform enabling patients to
enroll and participate in clinical trial activities. The
platform is the industry’s most comprehensive
solution for all aspects of site and remote-based
clinical research.
myMedidata encompasses all of the capabilities
of Medidata’s patient-facing solutions for
electronic patient consent and clinical outcomes
assessment (eCOA), collection of critical data
through wearable and other biosensors, COVID-19
symptom tracking, live video investigator/patient
visits, and enablement of hybrid and virtual trials.
Using myMedidata, patients can easily complete
forms, participate in video visits with their study
team, receive reminders and notifications for
study-related tasks, and access their results from
these patient-centric data capturing applications,
through one web-based, intuitive interface.

Highlights
- Single-destination patient portal designed with patient-centricity, which enables patients to use any online device to virtually learn, enroll and participate in clinical trial activities. It is the only patient portal platform which provides a holistic end-to-end solution from the recruitment of patients to the telemedicine visits and the return of patient data to the participant
- Complimentary COVID-19 Symptom Tracker-enabling patients on any active clinical study (regardless of therapeutic focus) to monitor and track their COVID-19 symptoms in order to better track their progression, remain active in any current clinical trials while providing more accurate trial data
- The latest release includes myMedidata LIVE, a web-based, live video conferencing capability that connects patients virtually with their clinical trial study staff, providing researchers and patients a way to engage in remote site visits within the platform of site and patient-facing technologies
View Website
 Back to Hall of Fame
Back to Hall of Fame
Medidata’s patient portal — myMedidata — is a
single-destination platform enabling patients to
enroll and participate in clinical trial activities. The
platform is the industry’s most comprehensive
solution for all aspects of site and remote-based
clinical research.
myMedidata encompasses all of the capabilities
of Medidata’s patient-facing solutions for
electronic patient consent and clinical outcomes
assessment (eCOA), collection of critical data
through wearable and other biosensors, COVID-19
symptom tracking, live video investigator/patient
visits, and enablement of hybrid and virtual trials.
Using myMedidata, patients can easily complete
forms, participate in video visits with their study
team, receive reminders and notifications for
study-related tasks, and access their results from
these patient-centric data capturing applications,
through one web-based, intuitive interface.
Highlights
- Single-destination patient portal designed with patient-centricity, which enables patients to use any online device to virtually learn, enroll and participate in clinical trial activities. It is the only patient portal platform which provides a holistic end-to-end solution from the recruitment of patients to the telemedicine visits and the return of patient data to the participant
- Complimentary COVID-19 Symptom Tracker-enabling patients on any active clinical study (regardless of therapeutic focus) to monitor and track their COVID-19 symptoms in order to better track their progression, remain active in any current clinical trials while providing more accurate trial data
- The latest release includes myMedidata LIVE, a web-based, live video conferencing capability that connects patients virtually with their clinical trial study staff, providing researchers and patients a way to engage in remote site visits within the platform of site and patient-facing technologies

View Website