Rave Companion
Medidata Solutions International Asia Pacific Pte. Ltd.
Rave Companion
Medidata Solutions International Asia Pacific Pte. Ltd.
 Back to Hall of Fame
Back to Hall of Fame
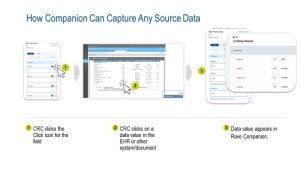
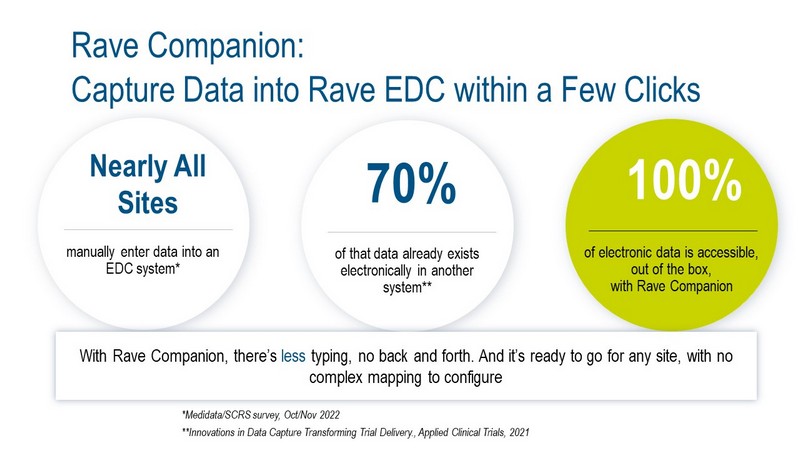
Medidata's Rave Companion reduces data re-entry
efforts for clinical trial sites, increases data quality, and
lowers monitoring costs by making it much simpler and
faster to get source data from other electronic systems/
documents, including EHR (electronic health record)
systems into Rave EDC. Rave Companion eliminates
the need for back-and-forth between windows and
dramatically reduces the need for retyping data from
another source into Rave EDC.
Unlike EHR-to-EDC integration approaches that require
point-to-point connections and complex mapping
between systems, Rave Companion is much simpler
and faster to implement, making it very scalable, and
it is not limited to EHR data. Unlike solutions that try
to completely automate the EHR to EDC data transfer,
with Rave Companion, the clinical trial site has complete
control of what data is s aved into Rave EDC.
One clinical trial site using Rave Companion saw the time
to complete a complex EDC form decrease by 44-times,
from 22 minutes spent on manual data entry to 0.5
minutes. By reducing the time spent on entering data
and resolving queries, trial site personnel can focus on
spending time on more valuable clinical research and
patient care tasks, and sponsors and CROs get access to
higher-quality data faster.

Dan Braga, the Vice President of EHR Solutions and Healthcare at Medidata.

Highlights
- Rave Companion is available to all sites and studies using Rave EDC with a simple set-up procedure for clinical trial site users.
- Rave Companion does not require protocol mapping and uses a multi-pronged approach to accessing patient health record data via Medidata Health Record Connect. Medidata Health Record Connect uses multiple industry standards to create clinical site connections. Once the site has been connected, it can be leveraged across any sponsor, any study, with no additional integration.
- Rave Companion uniquely assists the clinical trial site user in completing EDC forms, not only through the presentation of the patient’s structured health record data (via Medidata Health Record Connect) but also by completing other fields in the form while taking the form alongside any source application or document. Using Rave Companion, the user is assisted in completing the whole form, and not just the parts that are populated with EHR data.
View Website
 Back to Hall of Fame
Back to Hall of Fame
Medidata's Rave Companion reduces data re-entry
efforts for clinical trial sites, increases data quality, and
lowers monitoring costs by making it much simpler and
faster to get source data from other electronic systems/
documents, including EHR (electronic health record)
systems into Rave EDC. Rave Companion eliminates
the need for back-and-forth between windows and
dramatically reduces the need for retyping data from
another source into Rave EDC.
Unlike EHR-to-EDC integration approaches that require
point-to-point connections and complex mapping
between systems, Rave Companion is much simpler
and faster to implement, making it very scalable, and
it is not limited to EHR data. Unlike solutions that try
to completely automate the EHR to EDC data transfer,
with Rave Companion, the clinical trial site has complete
control of what data is s aved into Rave EDC.
One clinical trial site using Rave Companion saw the time
to complete a complex EDC form decrease by 44-times,
from 22 minutes spent on manual data entry to 0.5
minutes. By reducing the time spent on entering data
and resolving queries, trial site personnel can focus on
spending time on more valuable clinical research and
patient care tasks, and sponsors and CROs get access to
higher-quality data faster.


Highlights
- Rave Companion is available to all sites and studies using Rave EDC with a simple set-up procedure for clinical trial site users.
- Rave Companion does not require protocol mapping and uses a multi-pronged approach to accessing patient health record data via Medidata Health Record Connect. Medidata Health Record Connect uses multiple industry standards to create clinical site connections. Once the site has been connected, it can be leveraged across any sponsor, any study, with no additional integration.
- Rave Companion uniquely assists the clinical trial site user in completing EDC forms, not only through the presentation of the patient’s structured health record data (via Medidata Health Record Connect) but also by completing other fields in the form while taking the form alongside any source application or document. Using Rave Companion, the user is assisted in completing the whole form, and not just the parts that are populated with EHR data.
Dan Braga, the Vice President of EHR Solutions and Healthcare at Medidata.

View Website